ODFS®
Pace XL
Technology is an advanced system providing both Functional Electrical Stimulation (FES) and Neuromuscular Electrical Stimulation (NMES) for the treatment of upper and lower extremity dysfunction caused by upper motor neuron injuries such as:

• Multiple Sclerosis
• Stroke
• Incomplete Spinal Cord Injury
• Cerebral Palsy
• Traumatic Brain Injury
• Familial/Hereditary Spastic Paraparesis
• Parkinson’s Disease
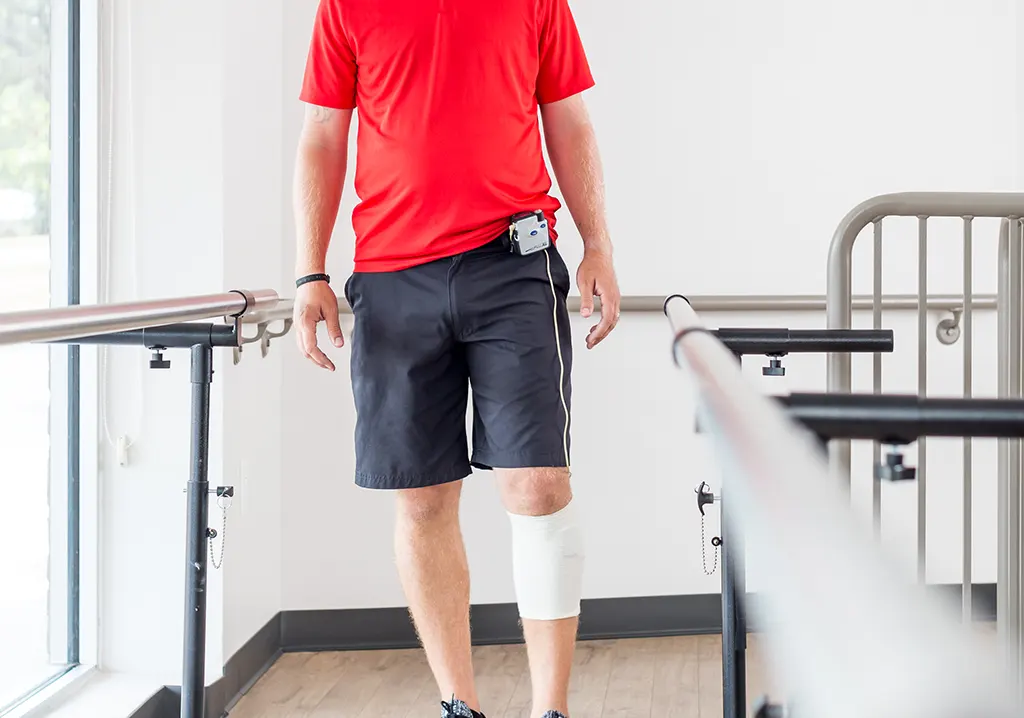
The ODFS® Pace XL is a single-channel neuromuscular stimulator, controlled by a wireless footswitch. Designed to correct drop foot in upper motor neuron conditions, the Pace XL also features protocols to address weakness, spasticity and/or other dysfunctions of the calf, thigh, gluteal, hand, forearm, arm and shoulder muscles; all cleared by the FDA.
Effective in Rehabilitation as Well as Every Day Life
The main purpose of the ODFS® Pace XL is to be a long-term mobility aide, however, the device can also be used as a therapeutic modality for gait training, tone management, strengthening, building endurance, neuromuscular re-education, etc. Using the ODFS” Pace XL in all stages of neuro-rehabilitation provides therapists a significant tool to attain positive patient outcomes:
• Improved ground clearance during swing
– Reduced tripping and falls
– Reduced compensatory activity
– Reduced effort of walking
– Increased walking speed
• Heel strike with eversión
– Improved loading response
– Greater stability in stance
• Greater range of mobility
• Reduced spasticity
• Long term therapeutic benefit
• Greater safety, confidence and independence while walking
• Greater social interaction and improved quality of life


Short and Long Term Rehabilitation Solutions
Drop Foot: Facilitate a natural dorsiflexion and eversión to clear the toes through swing; enable heel strike; promote control and stability into and through stance.
Calf: Encourage push-off and knee flexion at terminal stance.
Gluteals: Practice weight shifting and improve walking with cues for hip extension and/or abduction.
Hamstrings: Address knee hyperextension at initial contact.
Quadriceps: Practice weight transfer and increase knee extension during walking.
Triceps: Inhibit biceps spasticity and facilitate natural arm swing with walking.
Wrist & Finger Extensors: Strengthen extensor muscles, inhibit flexor spasticity and increase range of motion of the hand and wrist.
Shoulder Subluxation: Reduce shoulder pain and subluxation.
A New Level of Simplicity in FES/NMES Technology
Provide treatment and collect objective data with the push of a button.
- Program the Pace XL without a computer.
- Quickly set up and adjust program options.
- Easily switch between FES for gait and NMES treatment of the lower or upper extremity.
- Track steps taken (involved extremity), number of walking and exercise events, and total time spent on these activities.
- Assure stimulation for every step during walking, with the OML LINQ™ wireless footswitch.
- Position the control unit at the waist or on the leg, eliminating clothing restrictions.
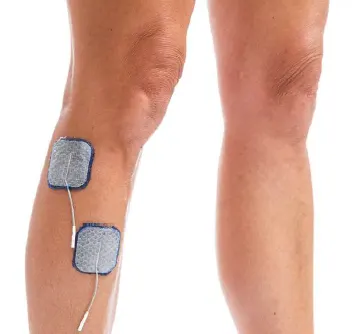
Drop Foot/Standard:
Lift toes with dorsiflexion and eversión
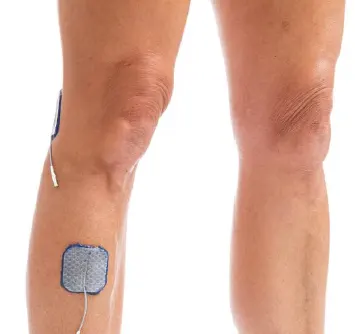
Drop Foot/Lateral Hamstring:
Lift toes and encourage hip/knee flexor withdrawal
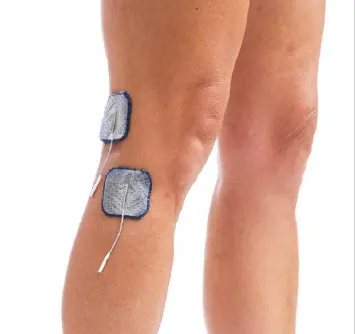
Drop Foot/Lateral Hamstring:
Lift toes and encourage hip/knee flexor withdrawal
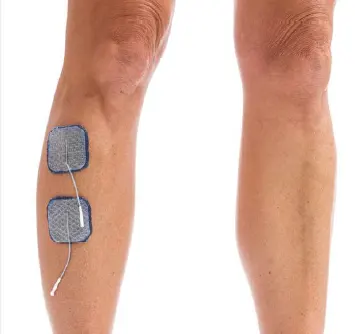
Drop Foot/Anterior Tibialis
Lift toes with increased dorsiflexion

Calf
Induce push-off and/or knee flexion at terminal stance
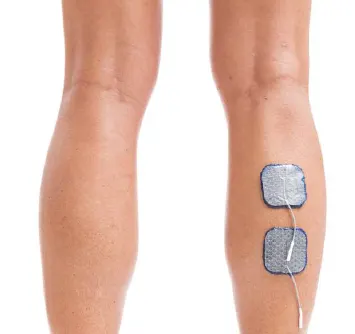
Calf
Induce push-off and/or knee flexion at terminal stance
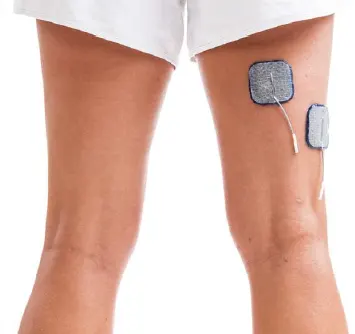
Hamstrings:
Promote knee flexion or prevent knee hyperextension
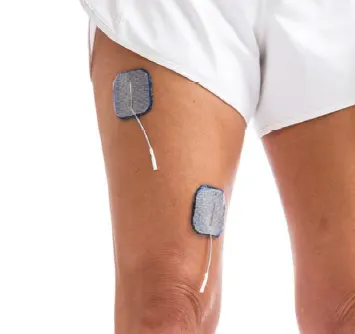
Quadriceps & Gluteals:
Fncourage knee or hip extension in stance
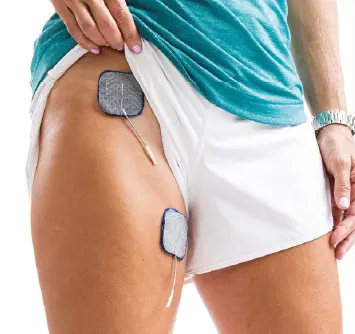
Hip Flexors:
Increase hip flexion

Forearm
Assist wrist and/or finger extension
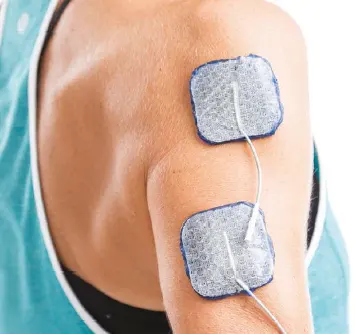
ForearmTriceps:
Inhibit biceps spasticity and/or facilitate natural arm swing
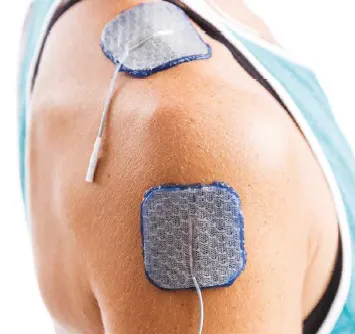
Shoulder:
Reduce subluxation and pain

Evidence Supporting FES

A randomized controlled trial of patients with chronic stroke using the ODFS® found;
• Gait speed increased 16% with the device.
• Physiological cost index (an estimate of the effort of walking) was reduced by 29%.
• Quadriceps spasticity decreased.
• Anxiety and depression were reduced.
• A significant positive cost benefit resulted from the treatment.
A subsequent audit of clinical services confirmed the RTC results and showed a training effect:
• Patients walked 27% faster with the device.
• They also walked 14% faster without it!
Two controlled studies of using ODFS® in people who had Multiple Sclerosis showed:
• FES users experienced 72% fewer falls than a control group who only received physical therapy.
• FES users reported a greater improvement in activities of daily living than the control group.
In multiple studies, the ODFS* has been shown to be a cost effective, long-term mobility aide.
• Adherence to treatment was 92% at 18 weeks and 86% at 1 year.
• Average use of the device was 5 years.
• 26% of device users were still benefitting from the device after 11 years.
